How AirClean Systems products can aid in USP <800> compliance
AirClean Systems products can provide compliance to USP 800 in the following areas:
The enclosures outlined below allow, with additional measures, a facility to become compliant to USP 800 requirements. These products are considered to be either Class I, Class II BSCs and LAFW's for use in compliance to USP 800 or relevant sections of USP 795 and USP 797.
What is USP <800> and Where Can I Find It ?
The United States Pharmacopeia (USP) General Chapter <800>, is a standard written to provide guidance when working with Hazardous Drugs (HDs), in an effort to mitigate worker exposure to HDs. This chapter has been published in the First Supplement to USP 39-NF 34.
Access to this standard can be purchased through the USP online store here: http://www.usp.org/products. We suggest the online subscription as this access keeps you up-to-date and allows for download capability of documents.
Who Does <800> Apply to ?
Section 1 of <800> states: "This chapter applies to all healthcare personnel who handle HD preparations and all entities that store, prepare, transport, or administer HDs (e.g., pharmacies, hospitals and other healthcare institutions, patient treatment clinics, physicians' practice facilities, or veterinarians' offices)."
To further illustrate this point, <800> outlines a few activities that can be considered as 'HD handling.' According to Section 1, "Handling HDs includes, but is not limited to, the receipt, storage, compounding, dispensing, administration, and disposal of sterile and nonsterile products and preparations.” Please note that <800> considers these as examples, and not the only forms of HD handling – i.e. production facilities are not exempt.
What is a HD According to <800> ?
As specified by Section 2 of <800>, "The NIOSH list of antineoplastic and other HDs provides the criteria used to identify HDs." If an entity chooses to handle HDs, they must maintain a list of HDs used that "must be reviewed at least every 12 months" or "whenever a new agent or dosage form is used."
Are There Any HD Exemptions ?
Section 2 of <800> states that "Some dosage forms of drugs defined as hazardous may not pose a significant risk of direct occupational exposure because of their dosage formulation (e.g., tablets or capsules…)". However, <800> does warn that "...dust from tablets and capsules may present a risk of exposure by skin contact and/or inhalation. An assessment of risk may be performed for these dosage forms to determine alternative containment strategies and/or work practices."
What HD Activities Should My Entity Look For ?
According to Section 3 of <800>, there are many “routes of unintentional entry of HDs into the body..." In order to help facilities and inspectors identify these unintentional routes of exposure, <800> provided a list of example activities with unintended exposure routes.
| Table 1. Examples of Potential Opportunities of Exposure Based on Activity | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Activity | Potential Opportunity of Exposure | ||||||||||
| Receipt |
|
||||||||||
| Dispensing |
|
||||||||||
| Compounding and Other Manipulations |
|
||||||||||
| Administration |
|
||||||||||
| Patient-care Activities |
|
||||||||||
| Spills |
|
||||||||||
| Transport |
|
||||||||||
| Waste |
|
||||||||||
How Does <800> Require My Entity to Mitigate HD Exposure ?
According to Section 5 of <800>, "Designated areas must be available for: Receipt and Unpacking, Storage of HDs, Nonsterile HD compounding (if performed by the entity) and Sterile HD compounding (if performed by the entity)." The standard then defines facility control requirements within each area, summarized below.
Receipt and Unpacking Requirements
Section 5.1 of <800> specifies, "Antineoplastic HDs and all HD APIs must be unpacked ... in an area that is neutral/normal or negative pressure relative to the surrounding areas.” In addition, "HDs must not be unpacked ... in sterile compounding areas or in positive pressure areas." For more information, please see Slides 23-24 & 91-94 of our <800> Summary Presentation.
Storage Requirements:
Section 5.2 of <800> outlines storage requirements for sterile and nonsterile HDs. According to <800> HDs should be stored in a separate area "...in a manner that prevents spillage or breakage if the container falls." For example an under-the-bench cabinet that "must meet applicable safety precautions, such as secure shelves with raised front lips." More information on how to store HD inventory is available in Slides 25-29 of our <800> Summary Presentation.
C-PEC (Enclosure) and C-SEC (Room) Requirements:
§ Section 5.3.1 - Nonsterile HD Compounding:
| Use | Optimal Primary and Secondary Control | Minimum ACPH | Limitations Primary and Secondary Control | Minimum ACPH | Notes for Limitations |
|---|---|---|---|---|---|
| Nonsterile HD Compounding | 12 |
C-PEC
Negative for HDI's
|
Appendix 2: Examples of Designs for Hazardous Drug Compounding Areas, (2016)
According to Section 5.3.1 of <800>, "Nonsterile HD compounding must be performed in a CPEC [an enclosure] that provides personnel and environmental protection such as a Class I BSC or Containment Ventilated Enclosure (CVE)." "The C-PECs used for manipulation of nonsterile HDs must be either externally vented ... or have redundant-HEPA filters in series." Finally, the "critical environment [of the powder enclosure] does not need to be ISO classified."
In regards to the C-SEC (the room that the enclosure is placed into), Section 5.3.1 of <800> states: "The C-SEC used for sterile and nonsterile compounding must: Be externally vented*, Be physically separated (i.e., a different room from other preparation areas), Have an appropriate air exchange (e.g. ACPH), Have a negative pressure between 0.01 and 0.03 inches of water column relative to all adjacent areas." *Published errata remove previous HEPA requirement.
Can I vent the C-SEC (the room) through the C-PEC (the powder enclosure) ?
According to Section 5.3 and 5.3.1 of <800>, "The C-PEC [the enclosure] must operate continuously if it supplies some or all of the negative pressure in the C-SEC [the room] or if it is used for sterile compounding." Therefore, the answer according to <800> is 'yes.' AirClean Systems can provide both vented and filtered enclosures for this process.
However, balances typically have problems taring out in enclosures that are externally vented and supply negative pressure to rooms. Why? This is a result of the venting process which causes the airflow to become more turbulent within the enclosure. This problem becomes amplified when connecting multiple hoods up to the same ductwork.
Furthermore, according to <800>, you can’t ever turn off or turn down your C-SEC (room) negative pressure if you vent your room through your hood. Turning down the Air Changes Per Hour (ACPH) at night or when the lab is unoccupied, will typically save an entity a surprising amount of HVAC cost in the long run. For more info on nighttime/unoccupied room ACPH see OHSA 1910.1450.
General construction requirements for both the C-PEC (the enclosure) and the C-SEC (the room) within Section 5.3.1 of <800> state that "...surfaces of ceiling walls, floors, fixtures, shelving, counters, and cabinets in the nonsterile compounding area must be smooth, impervious, free from cracks and crevices, and non-shedding." Essentially, there can be no paint chipping, no rust, no inserted slabs or work surfaces (crevices), etc.
§ Section 5.3.2 - Sterile HD Compounding:
| Use | Optimal Primary and Secondary Control | Minimum ACPH | Limitations Primary and Secondary Control | Minimum ACPH | Notes for Limitations |
|---|---|---|---|---|---|
| Sterile HD Compounding |
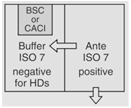 OR 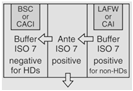
|
30 | 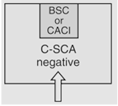 |
12 | Maximum BUD as described in <797> for segregated compounding area. |
 |
30 | If this design is in place, measures must be taken to avoid contamination of the positive-pressure buffer room. | |||
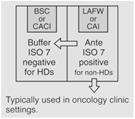 |
30 | Maximum BUD as described in <797>. |
Appendix 2: Examples of Designs for Hazardous Drug Compounding Areas, (2016)
Pursuant to § Section 5.3.2 of <800>, "Sterile HD compounding must be performed in a C-PEC that provides an ISO Class 5 or better air quality..." Furthermore, "All C-PECs [enclosures] used for manipulation of sterile HDs must be externally vented." This limits the user to operator & process protection products – such as: Class II BSCs B1 or B2, Class III BSCs, CACIs, etc.
Proposed updates to USP <797> divide Compounded Sterile Products (CSPs) into two categories based largely on facility design. The C-SEC (room) air requirements will differ depending on the Category of CSP that the user is trying to achieve (summarized below).
| Image 1. CSP Categories & C-SEC Requirements | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CSP Category | Sterile C-SEC (Room) Air Quality Requirement | Up-Fit Cost | Max BUD Time (with refridgeration) | HD Categories | |||||||
| Category 1 | Unclassified C-SCA | Low | 24 hours | Low & Medium Risk Only | |||||||
| Category 2 | ISO 7 Buffer Room + ISO7 Ante-Room | High | 45 days | All Legally Permitted | |||||||
For more information on C-SEC construction requirements, please see Slides 48-52 of our <800> Presentation. For information on proposed USP <797> updates to categorize CSPs and refine Beyond-Use-Date (BUD) Times, please visit: http://www.usp.org/usp-nf/notices/generalchapter-797-proposed-revision or ask to see our USP <797> Revision Presentation.
Sterile HD and Sterile Non-HD Compounding
For more information on C-SEC construction requirements, please see Slides 48-52 of our <800> Presentation. For information on proposed USP <797> updates to categorize CSPs and refine Beyond-Use-Date (BUD) Times, please visit: http://www.usp.org/usp-nf/notices/generalchapter-797-proposed-revision or ask to see our USP <797> Revision Presentation.
Sterile HD and Sterile Non-HD Compounding
| Use | Optimal Primary and Secondary Control | Minimum ACPH | Limitations Primary and Secondary Control | Minimum ACPH | Notes for Limitations |
|---|---|---|---|---|---|
| Both Sterile HD and Nonsterile HD Compounding | A separate room for sterile and nonsterile compounding is recommended. | 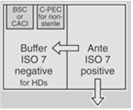
OR |
30 | For rooms used for both sterile and nonsterile compounding, particle-generating activity must not be peroformed when sterile comopunign is in process. C-PECs must be at least 1 meter apart. | |
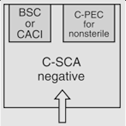
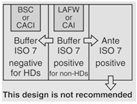
OR |
12 | Maximum BUD as described in <797> for segregated compounding area. | |||
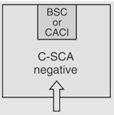
|
12 | Maximum BUD as described in <797> for segregated compounding area. |
Appendix 2: Examples of Designs for Hazardous Drug Compounding Areas, (2016)
According to <800> it is recommended that the entity use two separate rooms for sterile and nonsterile HD compounding. However, the standard does allow for configurations that combine sterile and nonsterile activity within the same room provided certain conditions are met.
- Enclosures "...must be placed at least 1 meter apart and particle-generating activity must not be performed when sterile compounding is in process."
- Nonsterile HD enclosure must be "...sufficiently effective that the [sterile HD buffer] room can continuously maintain ISO 7 classification throughout the nonsterile compounding activity."
For more information on Sterile HD and Nonsterile HD Compounding facility configurations and requirements, please see Slides 64-68 of our <800> Summary Presentation.
Deactivation, Decontamination, Cleaning and Disinfection Requirements:
According to Section 15 of <800>, "All areas where HDs are handled and all reusable equipment and devices must be deactivated, decontaminated, and cleaned." Section 15.2 addresses enclosures with removable work-surfaces/marble slabs, stating: "C-PECs may have areas under the work tray where contamination can build up. These areas must be deactivated, decontaminated, and cleaned at least monthly to reduce contamination level in the C-PEC."
After Deactivation, Decontamination, and Cleaning steps have been performed, Section 15 states that "sterile compounding areas and devices must be subsequently disinfected." When selecting a disinfectant, choose a product that meets the Section 15.4 requirement of "inhibiting or destroying microorganisms." For example, AirClean Systems offers Solucide®, a broadspectrum disinfectant spray and an EPA registered hard surface cleaner, disinfectant, and deodorizer for this purpose. For more information on Deactivation, Decontamination, Cleaning and Disinfection requirements, please see Slides 106-111 of our <800> Summary Presentation
Responsibilities of Personnel Handling HDs:
According to Section 4 of <800>, "Each entity must have a designated person that is qualified and trained" to develop and implement "appropriate procedures," oversee "entity compliance," ensure "competency of personnel," ensure "environmental control of the storage and compounding areas" and understand risks & responsibility to report potential hazards. This person "Must also be responsible for the oversight and monitoring of the facility and maintaining reports of testing/sampling performed in facilities and acting upon the results."
In addition, "All personnel who handle HDs are responsible for understanding the fundamental practices and precautions and for continually evaluating these procedures and the quality of the final HDs to prevent harm to patients, minimize exposure to personnel, and minimize contamination of the work and patient-care environment."
Additional Handling & Precautionary Procedures Outlined in <800>:
- Section 6. Environmental Quality and Control Sampling
- Section 7. Personal Protective Equipment Requirements
- Section 8. Hazard Communication Programs
- Section 9. Personnel Training
- Section 11. Labeling, Packaging, Transport and Disposal
- Section 12. Dispensing Final Dosage Forms
- Section 13. Compounding
- Section 14. Administering
- Section 16. Spill Control
- Section 17. Documentation and Standard Operating Procedures
- Section 18. Medical Surveillance
For your convenience, we have summarized all Sections of <800> in our <800> Summary Presentation. Should you have any questions or concerns, we're more than happy to help. Please contact our AirClean Systems team at: +1 919.255.3220
Glossary of Terms*
"Ante-room: An ISO Class 7 or cleaner room where personnel hand hygiene, garbing procedures, and other activities that generate high particulate levels are performed. The anteroom is the transition room between the unclassified area of the facility and the buffer room."
"Beyond-use date (BUD): The date or time beyond which a compounded preparation cannot not be used and must be discarded (see <795> and <797>). The date or time is determined from the date or time when the preparation was compounded."
"Buffer room: A type of C-SEC under negative pressure that meets ISO Class 7 or better air quality where the C-PEC that generates and maintains an ISO Class 5 environment is physically located. Activities that occur in this area are limited to the preparation and staging of components and supplies used when compounding HDs."
"Containment primary engineering control (C-PEC): A ventilated device designed and operated to minimize worker and environmental exposures to HDs by controlling emissions of airborne contaminants through the following:
- The full or partial enclosure of a potential contaminant source
- The use of airflow capture velocities to trap and remove airborne contaminants near their point of generation
- The use of air pressure relationships that define the direction of airflow into the cabinet
- The use of HEPA filtration on all potentially contaminated exhaust streams"
"Containment secondary engineering control (C-SEC): The room with fixed walls in which the C-PEC is placed. It incorporates specific design and operational parameters required to contain the potential hazard within the compounding room."
"Containment segregated compounding area (C-SCA): A type of C-SEC with nominal requirements for airflow and room pressurization as they pertain to HD compounding."
"Hazardous drug (HD): Any drug identified by at least one of the following criteria:
- Carcinogenicity, teratogenicity, or developmental toxicity
- Reproductive toxicity in humans
- Organ toxicity at low dose in humans or animals
- Genotoxicity or new drugs that mimic existing HDs in structure or toxicity"
"Supplemental engineering control: An adjunct control (e.g., CSTD) that may be used concurrently with primary and secondary engineering controls. Supplemental engineering controls offer additional levels of protection and may facilitate enhanced occupational protection, especially when handling HDs outside of primary and secondary engineering controls (e.g., during administering)."
"Unclassified space: A space not required to meet any air cleanliness classification based on the International Organization for Standardization (ISO)."
*Glossary terms quoted from:
United States Pharmacopeia 39, National Formulary 34 (USP), General Chapter 800, Glossary, (2016)
Disclaimer: The information provided here is the opinion of AirClean® Systems and by no means is an exact interpretation or expert opinion as to each individual healthcare provider facility. The information expressed in this document is for review and consumption by healthcare professionals that are charged with ensuring proper engineering safety controls for workers so as to maintain both patient and employee safety when dealing with HDs.
